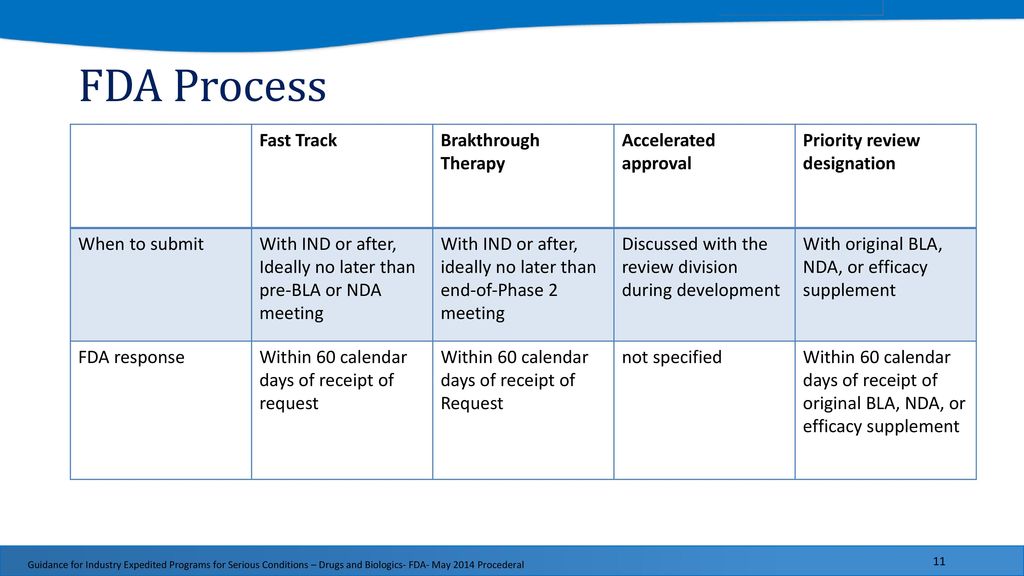
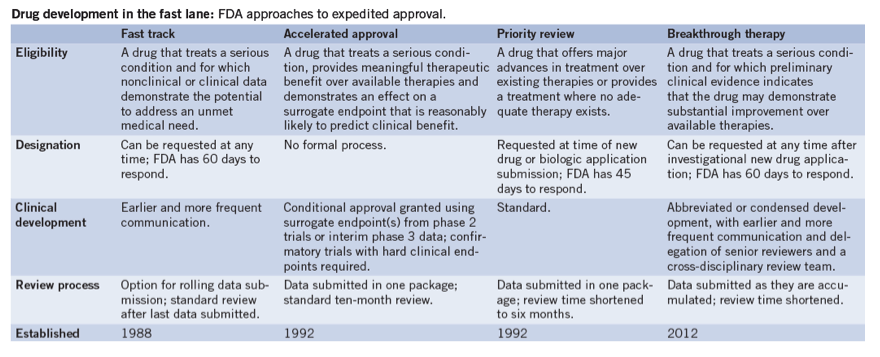
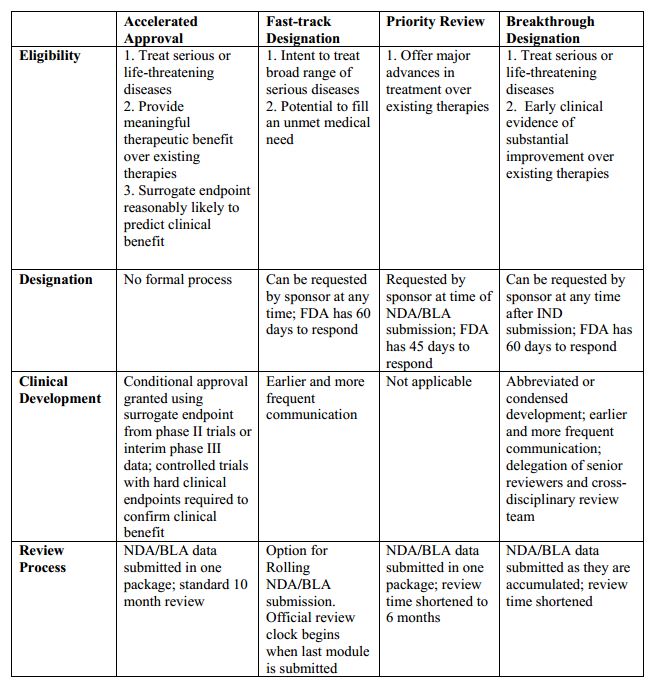
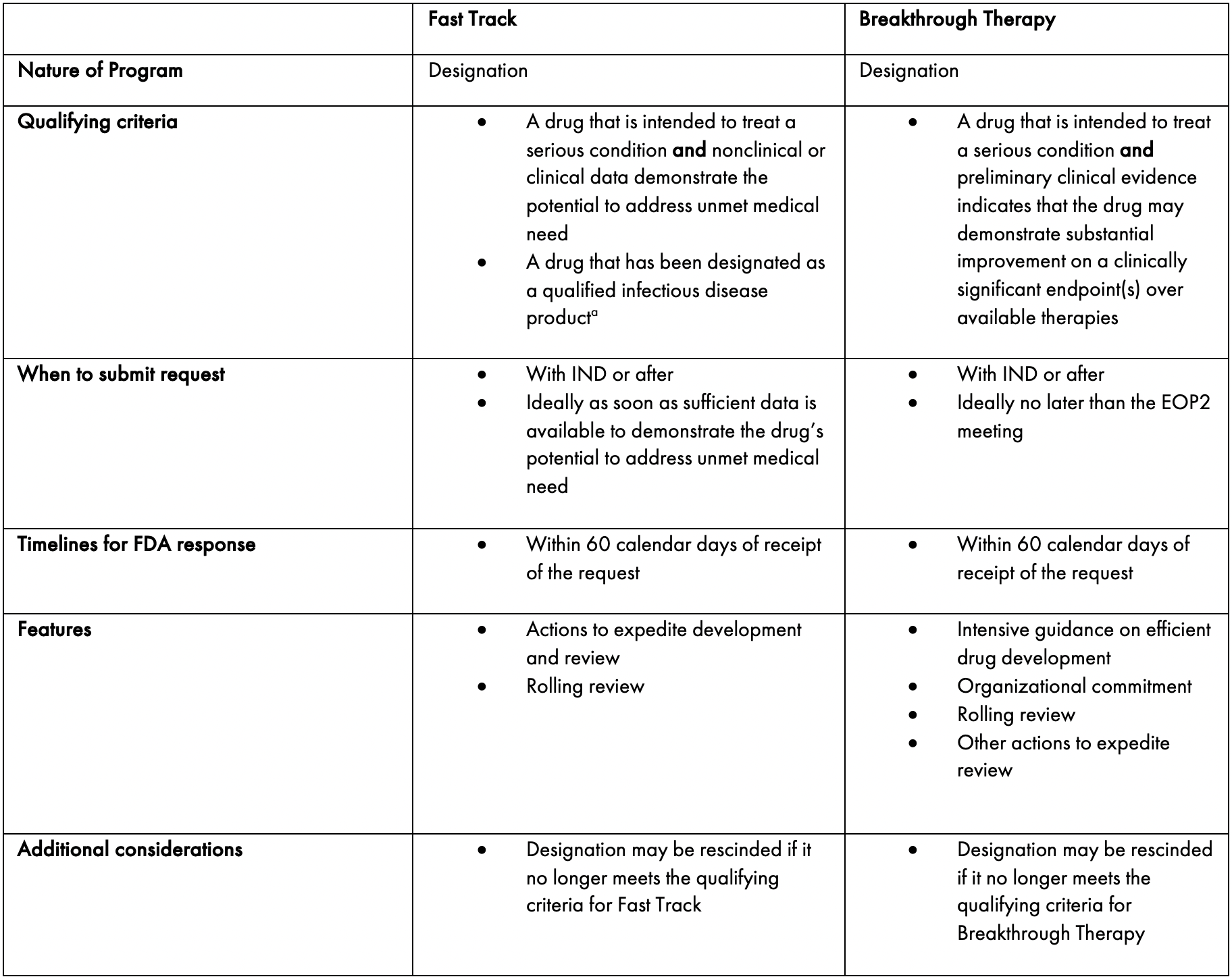
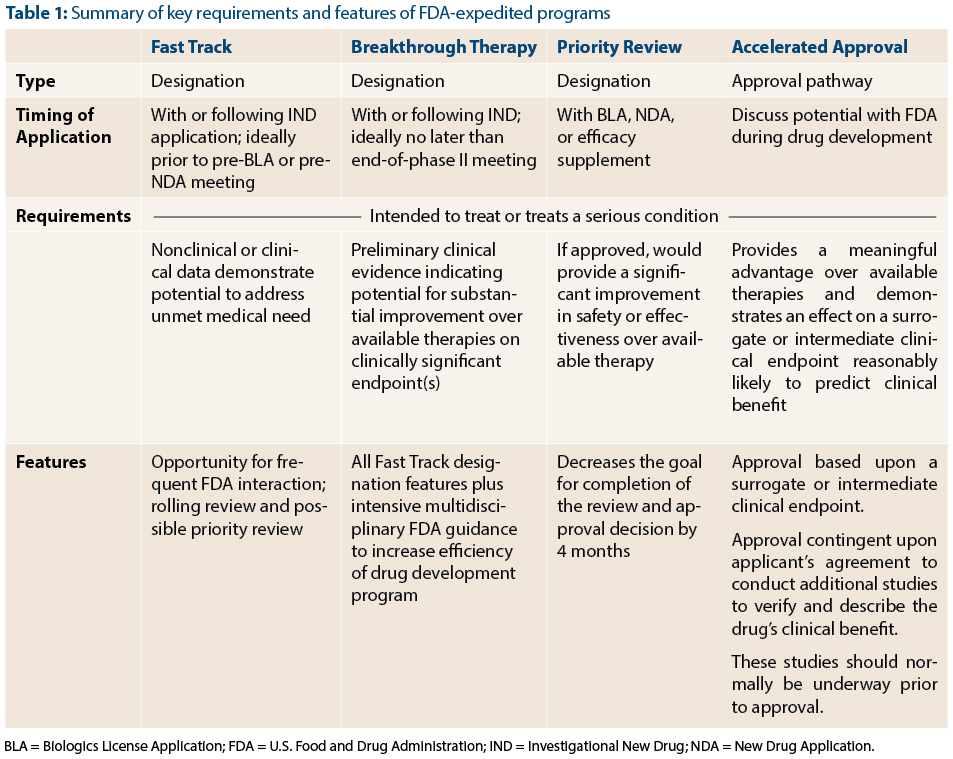
FDA efficiency for approval process of COVID-19 therapeutics | Infectious Agents and Cancer | Full Text

Table 2 from Expediting drug development--the FDA's new "breakthrough therapy" designation. | Semantic Scholar
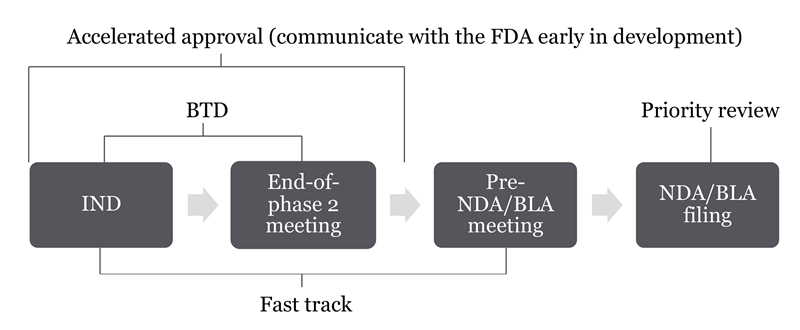
FDA Unveils Considerations for Rescinding Breakthrough Therapy Designation // Cooley // Global Law Firm
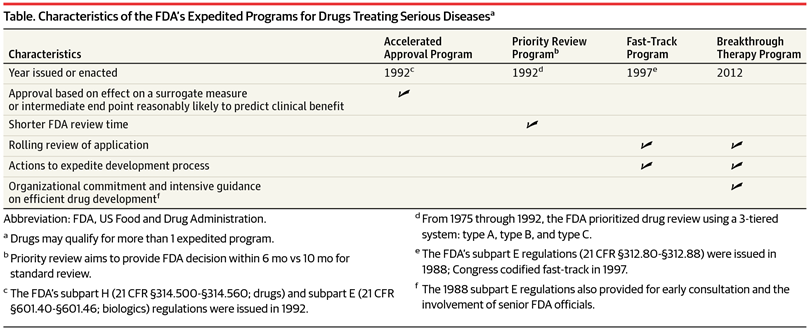
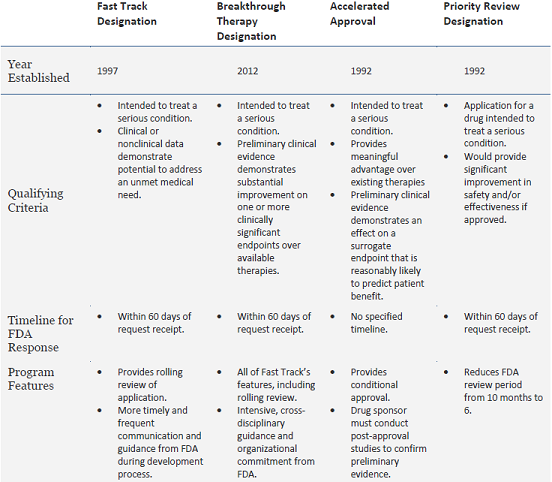
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB) | Seeking Alpha
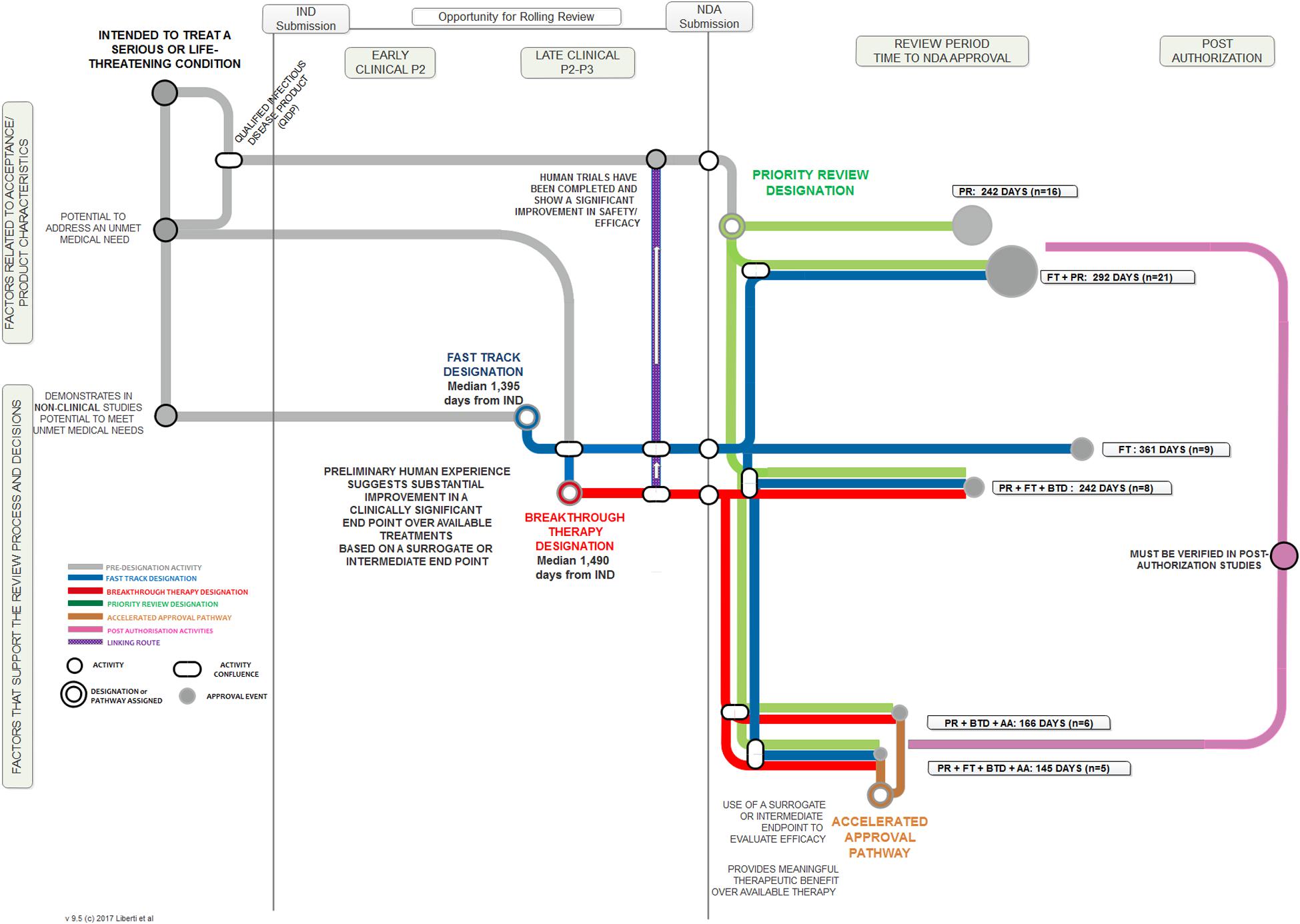
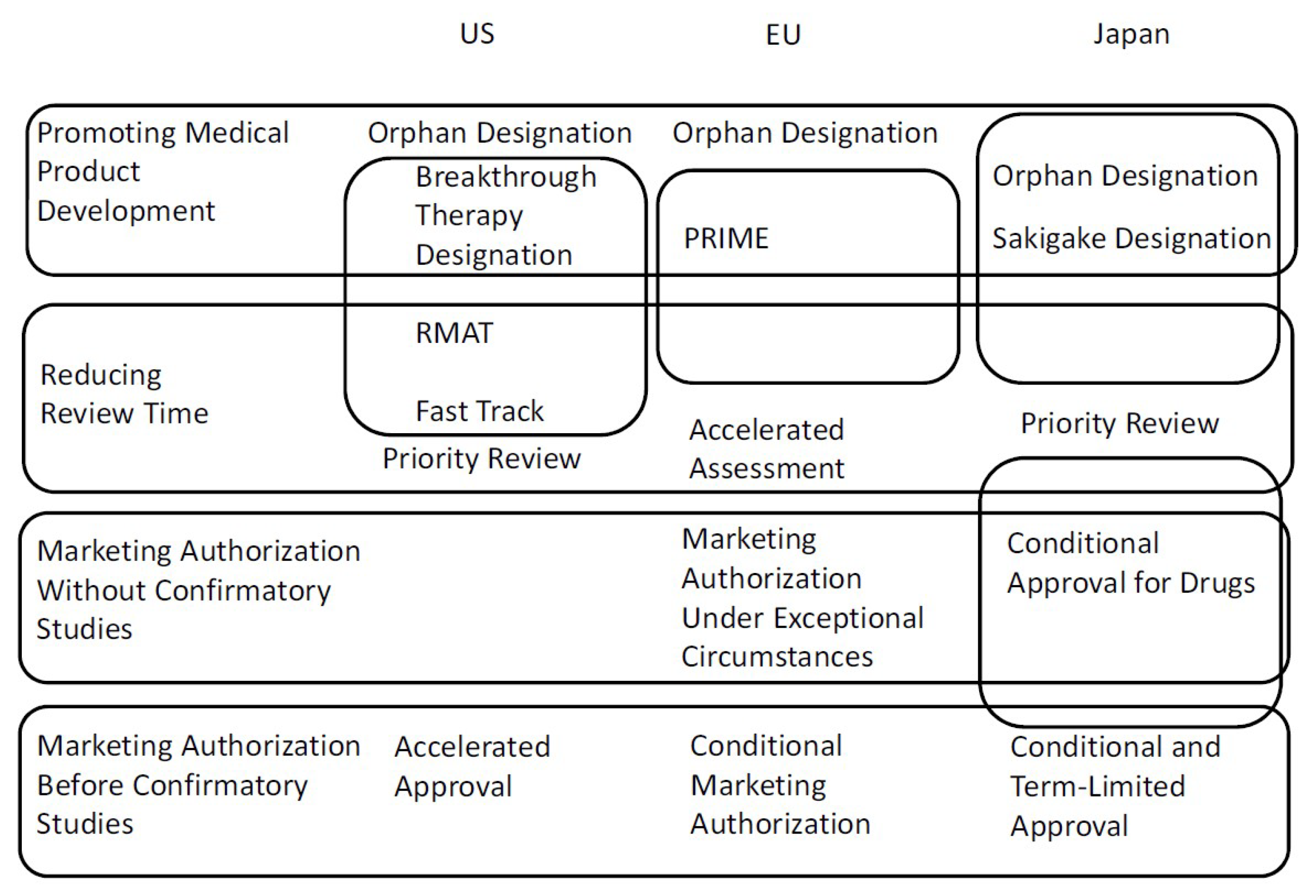
Frontiers | FDA Facilitated Regulatory Pathways: Visualizing Their Characteristics, Development, and Authorization Timelines
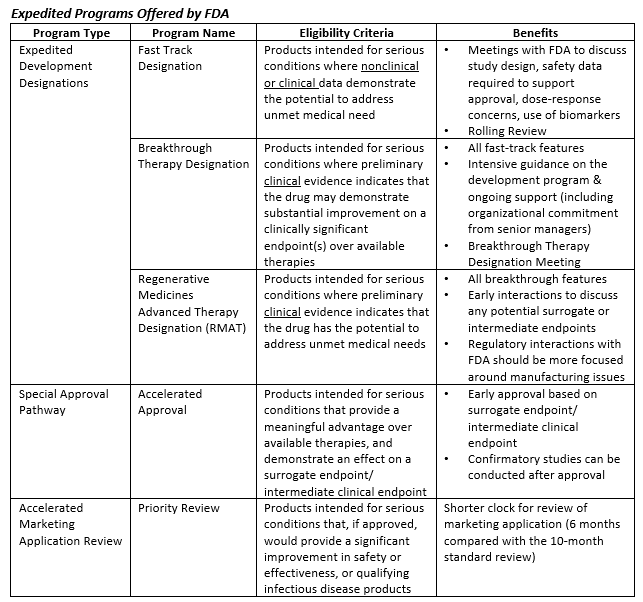
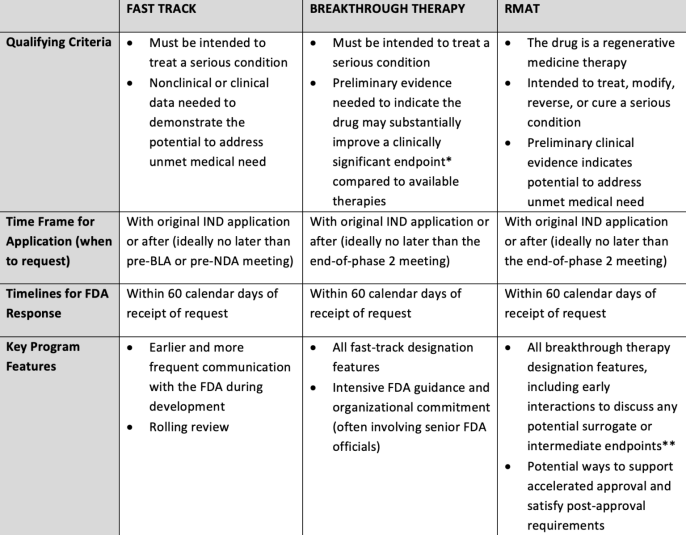
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group