First Patient Enrolled in Investigational Study of the GORE EXCLUDER Conformable AAA Endoprosthesis with ACTIVE CONTROL System | BioSpace

The schema of endovascular procedure A) The true lumen was compressed... | Download Scientific Diagram

Schematic representation of the endovascular steps employed in this... | Download Scientific Diagram
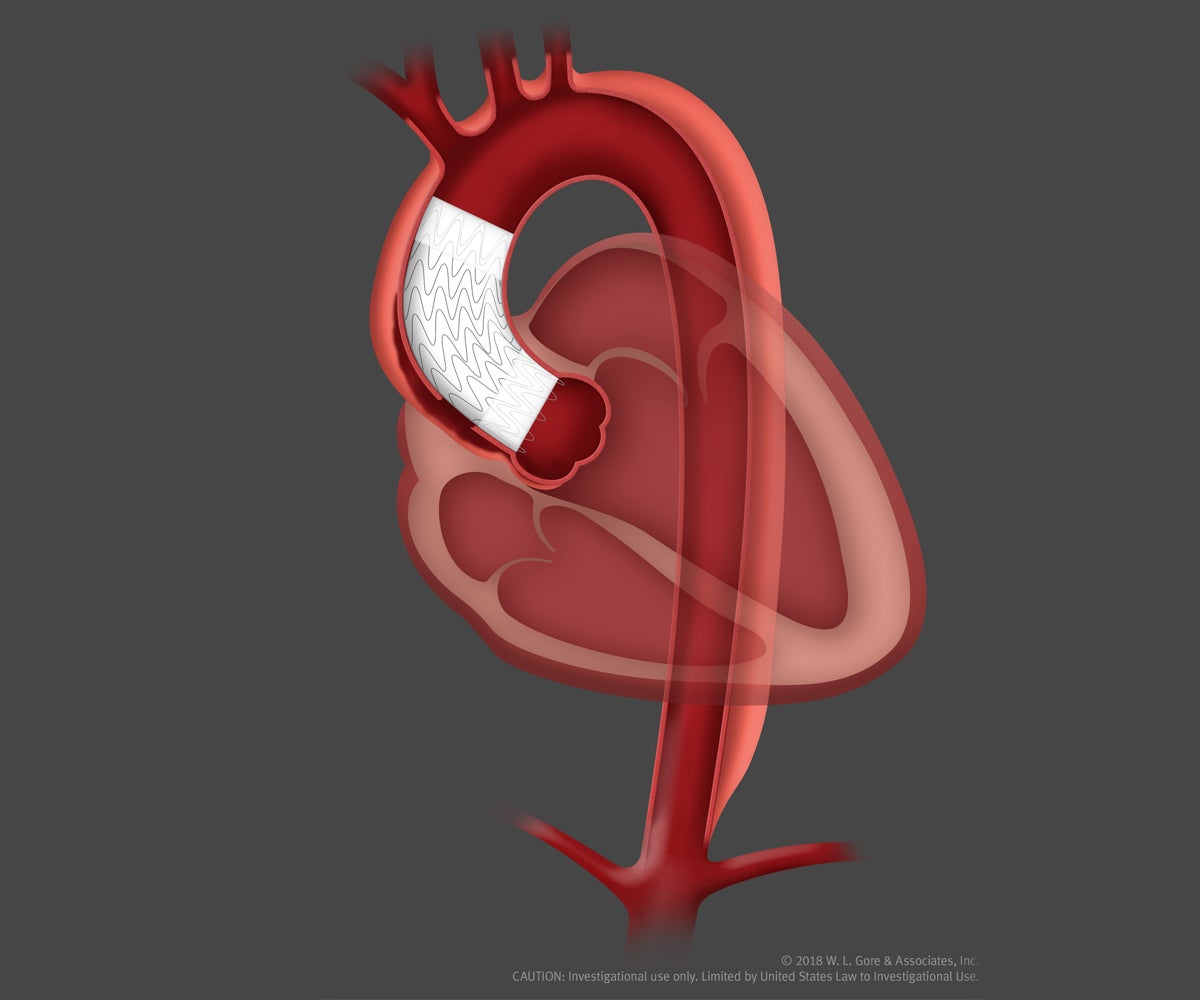
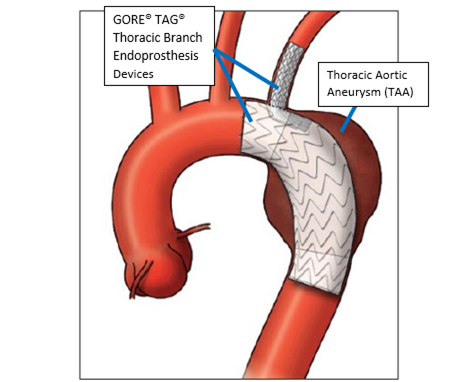
Gore Announces Successful Patient Implant of Endovascular Stent Graft for the Ascending Aorta | Gore

Use of retrograde left subclavian branch portal of Gore TAG thoracic branch endoprosthesis for physician-modified fenestrated branched endovascular repair of thoracoabdominal aortic aneurysm - Journal of Vascular Surgery Cases, Innovations and Techniques

Early Experience Using the GORE® TAG® Conformable Thoracic Stent Graft With ACTIVE CONTROL System: Why It Is My Preferred TEVAR Device - Endovascular Today

Gore Medical Products Division on LinkedIn: GORE RECEIVES FDA APPROVAL FOR BREAKTHROUGH ENDOVASCULAR DEVICE IN COMPLEX…

Figure 1 from Tested by Time The story of the evolution of endovascular aneurysm repair and the GORE ® EXCLUDER ® Device | Semantic Scholar

GORE® EXCLUDER® AAA Endoprosthesis Percutaneous Endovascular Aneurysm Repair Case Video (Dennis Gable, MD) – Vascupedia

GORE, PAHR060201E, VIABAHN, Endoprosthesis, with PROPATEN Bioactive Surface, 6mm x 2.5cm 7Fr, with radiopaque markers, 0.035" Guidewire Compatibility
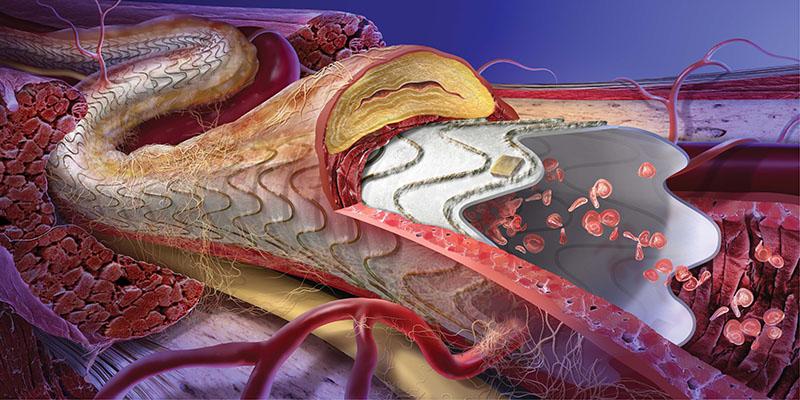
Gore Receives FDA Approval for Endovascular Treatment of In-stent Restenosis with the Gore Viabahn Endoprosthesis | DAIC